2-A. Chemical Principle (I)
π*-σ* Hyperconjugation
As seen in butadiene, the phenomenon in which two or more π orbitals interact and delocalize π electrons is called conjugation. Hyperconjugation is an extension of this concept to the interaction between the σ orbital of C-H bond and the π orbital of C-C bond. In the case of toluene, the σ orbital of C-H and the π* orbital of the benzene ring interact to stabilize. All of these are concepts for interactions in the ground state.
Prof. Masaaki Fujii (at that time, Waseda Univ.) informed that the internal rotation barriers of the methyl group of toluene derivatives behave completely differently in the ground and the excited states. In fact, even the stable orientation of the methyl group occasionally change in the excited state. Although the energy barrier of the internal rotation of the methyl group is a considerably small value of several tens to several hundreds of cm-1, it could be reproduced reasonably at the HF level. The frozen orbital analysis (FZOA) clarified that the difference in the internal rotation barriers of the methyl group between the ground and the excited states is brought about from the orbital energy difference between HOMO and LUMO, and further, the orbital energy of LUMO (Fig. 2-A-1). When focusing on LUMO, we discovered a special interaction between the σ* orbital of C-H bond and the π* orbital of the benzene ring, which leads to stabilization between the H atom and the C atom at the ortho position (Fig. 2-A-2). This special interaction seen in the excited state was named π*-σ* hyperconjugation. After we theoretically proposed this concept, confirmation by experimental studies has also been reported.
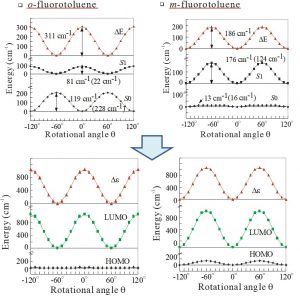
Fig. 2-A-1
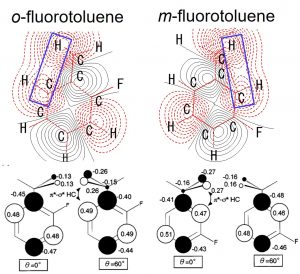
Fig. 2-A-2
Key Literature
<π*-σ* Hyperconjugation>
- H. Nakai, M. Kawai, “Nature of the change in the rotational barrier of the methyl group due to S0 → S1 excitation”, Chem. Phys. Lett., 307, 272 (1999).
- H. Nakai, M. Kawai, “π*-σ* Hyperconjugation mechanism on the rotational barrier of the methyl group (I): Substituted toluenes in the ground, excited, and anionic states”, J. Chem. Phys., 113, 2168 (2000).
- H. Nakai, Y. Kawamura, “π*-σ* Hyperconjugation mechanism on the rotational barrier of the methyl group (II): 1- and 2-methylnaphthalenes in the S0, S1, C0, and A1 states”, Chem. Phys. Lett., 318, 298 (2000).
- Y. Kawamura, T. Nagasawa, H. Nakai, “π*-σ* Hyperconjugation mechanism on the rotational barrier of the methyl group (III): methyl-azabenzenes in the ground, excited, and anionic states”, J. Chem. Phys., 114, 8357 (2001).
