Research contents
Keywords
Organic Synthesis Chemistry, Asymmetric Reaction Chemistry, Organometallic Chemistry
Reserch Outline
The development of a new and practical protocol for synthesizing complex molecules, in particular functional and biologically active compounds, is a major topic in organic chemistry. Most of these compounds have multi-chirality, including central, axial, planar, and helical chiralities. Thus, there is strong interest in efficient methods for asymmetric synthesis. Among the various approaches to generating chirality, enantioselective and catalytic cycloaddition is the most atom-economical and reliable, providing chiral multi-cyclic compounds along with carbon-carbon bond formations. Transition metal complexes, which operate as chiral catalysts, coordinate directly to reaction sites, such as alkene and alkyne, and the choice of appropriate chiral ligands (usually organic compounds) may realize high chemo- and stereloselectivity. Our focus is on the study of unique and practical carbon-carbon forming reactions.
Reseach Examples
(1) Generating axial chiralities:
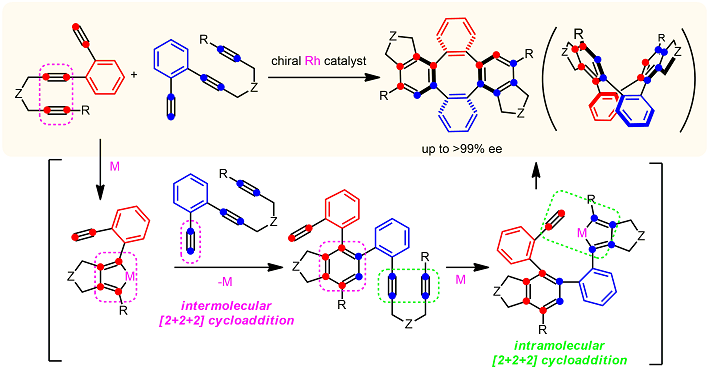
Asymmetric [2+2+2] cycloaddition of three alkyne moieties
An enantioselective [2+2+2] cycloaddition was achieved using a chiral iridium catalyst:
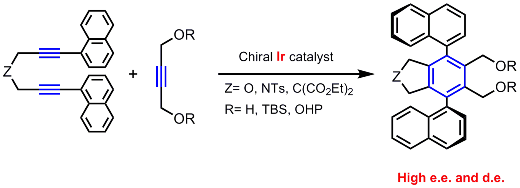
The intermolecular coupling proceeded efficiently between diynes,
possessing various tethers and aryl substituents on their alkyne termini,
and monoalkynes, possessing oxygen and/or nitrogen functionalities at the propargylic position(s).
Almost perfect enantio- and diastereoselectivities could be achieved
and various axially chiral teraryl compounds were obtained.
Consecutive reactions using poly-ynes with multi-diyne moieties as substrates
provided polyaryl compounds with up to eight axial chiralities in a pot.
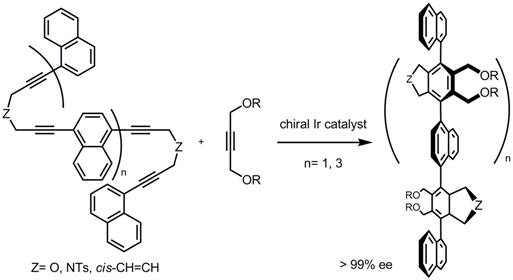
Tetraphenylene is a structurally unique saddle-shape molecule in which four benzene rings
are ortho-annelated to construct an eight-membered ring system.
The benzene rings cannot be positioned in a plane; rather, they must be arranged above and below.
We also achieved the first highly enantioselective and catalytic synthesis of chiral substituted tetraphenylenes
by consecutive inter- and intramolecular [2+2+2] cycloaddition of triynes.
(2) Generating asymmetric quaternary carbon atoms:
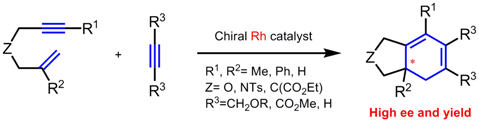
Asymmetric cycloaddition between an enyne moiety and an unsaturated moiety
Intermolecular [2+2+2] cycloaddition between 1,6-enyne, possessing various substituent (R2) on their alkene moiety,
and monoalkynes provide a chiral bicyclic 1,3-diene with quaternary carbon stereocenter at the fused ring position.
The product was obtained with excellent enantioselectivity by chiral rhodium catalyst.
This method is a new aproach of construction of quaternary carbon stereocenter via cycloaddition.
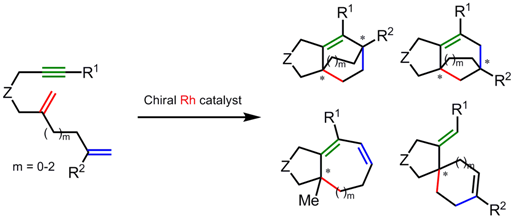
We also examined various 1,n-diene-ynes (n= 4-6) in cycloaddition as branched substrates using chiral Rh catalysts. The reaction pathways could be perfectly controlled by substituents on alkyne terminus and alkene moiety: tricyclic compounds with bridged structures, bicyclic compounds with medium ring systems, and spirocyclic compounds were provided selectively. All compounds possess asymmetric quaternary carbon atom(s) and were obtained with almost perfect enantioselectivity.
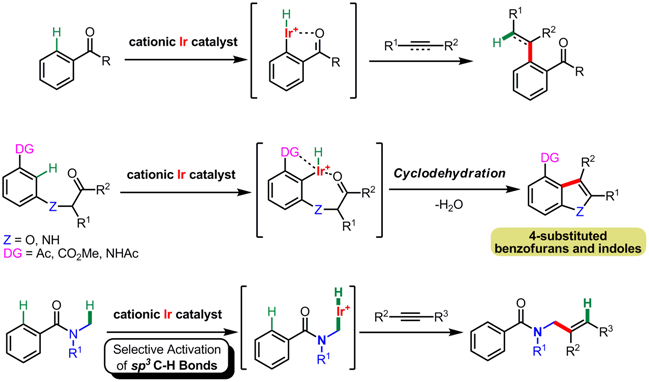
(3) C-H bond activation by cationic Ir catalysts
Transition metal-catalyzed inactive carbon-hydrogen bond functionalization is
an attractive process from both synthetic and atom-economical perspectives,
making it possible to eliminate the pre-activation step of substrates and reducing waste by-products.
Cationic Ir catalysts realized various types of synthetic transformations initiated by C-H bond.
We cleaved the Csp2-H bond adjacent to the carbonyl group on the aromatic ring
and reacted it with alkynes to generate alkenylated products.
Intramolecular reaction with carbonyl moiety could be possible and benzoheteroles,
which are ubiquitous compounds in nature, were obtained.
The even more stable Csp3-H bond was also cleaved,
and the reaction of amides and alkynes resulted in substituted allyl amides.