1-E. Practical condensed-phase free energy calculation method
HSM
Most of organic syntheses are solution reactions using various solvents. Continuum dielectric models are widely used for quantum chemistry calculations of molecules in solution. In the continuum dielectric model, the solute molecules (and a few solvent molecules that have a direct interaction with the solute) are treated quantum-chemically, and the bulk solvent is treated as a continuum. In the chemical reaction, the effect of temperature is indispensable. In the standard quantum chemistry calculations, thermodynamic quantities are estimated by assuming the nuclear motion as an ideal gas. Based on the assumption of the ideal gas, however, the entropy term for the solvent molecules treated in the continuum dielectric model is overestimated. Nakai’s Group has developed a harmonic solvation model (HSM) that approximates the effects of translation and rotation of molecules in solution with a harmonic oscillator (Fig. 1-E-1). Various condensed-phase properties such as boiling point, heat of evaporation, and standard hydrogen electrode were well demonstrated by using the HSM.
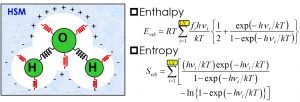
Fig. 1-E-1
Key Literature
<HSM>
- H. Nakai, A. Ishikawa, “Quantum chemical approach for condensed-phase thermochemistry: Proposal of a harmonic solvation model”, J. Chem. Phys., 141, 174106 (2014).
<Review (in Japanese)>
- H. Nakai, “調和溶媒和モデル(HSM)を用いた凝縮系の自由エネルギー計算”, J. Comput. Chem. Jpn., 16, 83 (2017).
