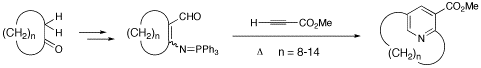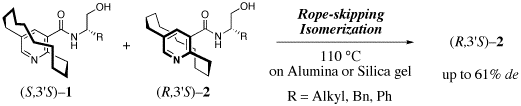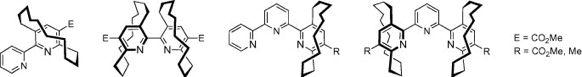|
Recent research projects
|
See also "Research Profile" edited by Faculty of Science & Engineering, Waseda University)
|
|
|
|
(S)-[10]parapyridinophane
|
(R)-[10]parapyridinophane
|
1. Planar-chiral molecules:
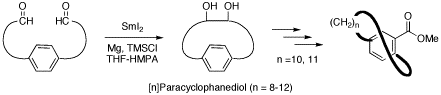
Synthetic studies on planar-chiral cyclophane molecules are one of our major interests. We have accomplished a synthetic methodology for preparation of pyridinophanes (pyridine-type cyclophanes) by using novel pyridine-formation reactions that we originally designed. Our recent efforts are concentrated on development of efficient bridge formation reactions for synthesizing functional cyclophane molecules via metal-mediated intramolecular cyclization such as pinacol coupling reactions and oxidative diyne coupling reactions.
2. Dynamic stereocontrol:
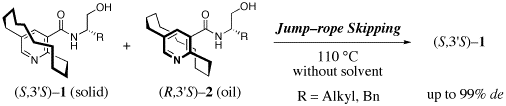
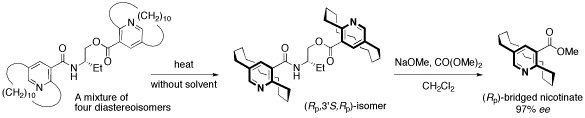
Stereocontrol of planar-chiral molecules is another research interest. Dynamic rope-skipping isomerization alternating planar-chirality was found to be very useful strategy for stereocontrol of Ågjump-rope moleculesÅh with accumulation of planar chirality. Crystallization-induced asymmetric transformation (CIAT) and adsorption-induced asymmetric transformation (AIAT) are two major methodologies for exploration of the efficient dynamic resolution. We have also demonstrated that such a method was applicable to synchronized stereocontrol of two planar-chiral units: CIAT of bispyridinophane derivatives afforded a single compound from among a mixture of four stereoisomers.
3. Planar-chiral molecules for asymmetric reactions:
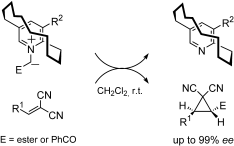
We are running a couple of research projects regarding synthesis of novel planar-chiral catalysts or ligands and their application to organic synthesis. Our target molecules are (i) planar-chiral pyiridinium ylides for enantioselective cyclopropanation reactions, (ii) planar-chiral analogs of bipyridine and terpyridine ligands, (iii) quarternary ammonium salts having cyclophane and pyridinophane units as chiral sources for asymmetric induction.
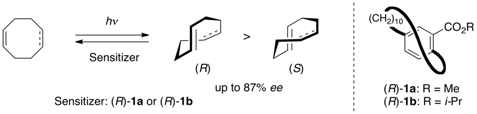
In collaboration with Prof. Yoshihisa Inoue and his coworkers, photochemical planar-to-planar chirality transfer was investigated by using (R)-[10]paracyclophane-12-carboxylates as a planar-chiral sensitizer and (Z)-cyclooctene and (Z,Z)-1,5- cyclooctadiene as prochiral substrates. The planar-chiral sensitizer effected asymmetric induction to the corresponding planar-chiral (E)- and (E,Z)-isomers in up to 44% ee and 87% ee, respectively, the latter of which being the highest ever reported for a sensitized photochirogenic reaction.
4. Artificial coenzyme and biomimetics:
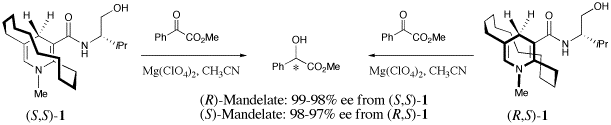
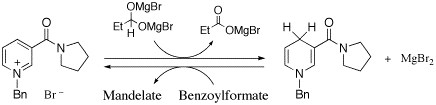
Development of new redox systems mimicking coenzyme NAD+/NADH is a current target of our research. Highly enantioselective and stereospecific reactions of pyruvate analogs are under investigation with originally desiged bridged NADH model compounds. Further research interests are examination of artifical biological redox in aerobic glycolysis; both glycelaldehyde-3-phosphate dehydrogenase-type and lactate dehydrogenase-type of biomimetic reactions.
To Top Page